| |
|||||||||
| |
|
|
|
|
|
|
|
||
| Home | About | Literature | Site Map | MSDS | Contact | Engineers | Shows | Links | Samples | RFQ | Catalog Numbers |

Hazard Communication . . . Right to Know . . . Permissible Exposure Limits . . . and that's just the beginning. It would be great if there weren't any "hazardous" substances, and thus no need to worry about regulations or safe use methods.
"Great," but not "real." At home or in the workplace, exposure to hazardous chemicals is frequent. Gassing up the car, washing the dishes, fertilizing the lawn, or painting the porch--these routine tasks are repeated countless times each day. Each involves exposure to chemicals with hazards.
Realistically, we must learn how to use or specify the use of workplace chemicals to minimize personal risk. But how do we know what the problems and hazards of a particular workplace material are, or what we can do about them?
This issue of "TeleTopics" will discuss chemical hazards and safety and, specifically, "toxicology" of workplace chemicals.
How much is Too Much?
Toxicology is simply the "science of poisons." If you look at toxicology data for baking soda, table salt, and cyanide, you may be surprised to find that all of them can poison you--all have "lethal doses"!!
However, as you might suspect, the cyanide is extremely toxic, and can be fatal at very low exposure levels. The two food materials have very low level toxicity, and large quantities are required for adverse systemic effects.
We've discovered the first important fact about chemical toxicity. All chemicals are somewhat toxic. What's important is the "degree" or "severity" of the toxicity. It's this "degree" we need to know to assess the hazard and to develop sage material handling procedures.
Taste, Touch, and Smell
Another important factor is the method of exposure to a specific workplace chemical. Most of us think of "poison" as being eaten or ingested (oral). However, toxic materials can also enter the human body through the skin (dermal) or via the lungs (inhalation).
In electrical construction, inhalation and dermal exposures are often the primary concern. Most field workers know better than to eat workplace materials but may not think about problems from smelling or touching them.
Where to Start
What we need is information on both "exposure" and "severity" for workplace chemicals. This information should be on a document called the MSDS (Material Safety Data Sheet) for the specific chemical. This MSDS is available from the chemical manufacturer. It provides information on toxicity, exposures, and first aid. The MSDS also contains information on fire hazard, chemical incompatibilities, disposal, and shipping.
Unfortunately, some MSDS sheets are not very complete and provide only marginally adequate information to help develop safe use procedures. A thorough MSDS sheet, on the other hand, contains lots of information, but does not interpret it for your specific situation and end use. So how can you interpret the MSDS?
Terrifying Terminology
Because an MSDS provides data to cover a broad variety of product use or misuse, some of the information supplied can sound downright scary.
One term often found on an MSDS is an "LD50"--which is the (lethal) dose required to produce a 50% test animal death rate. This quantity of material would (orally, dermally, intravenously, etc.) statistically result in the death of 50% of a group of test animals (usually rats, mice or guinea pigs). LD50 data is presented as a weight of the chemical to the weight of the animal, most commonly in milligrams of substance per kilogram of body weight (mg/kg).
Listed below are LD50 data on some familiar chemicals. A common use for the substance is also provided.
| Material/Use | LD50 | % of Body Weight |
|---|---|---|
| Curare (poison arrows) | (oral-rabbit) 270 mg/kg | .027% |
| Sodium Cyanide (mtl. finishing) | (oral-rat) 6.444 mg/kg | .00064% |
| Baking Soda (baking) | (oral-rat) 4,220 mg/kg | .42% |
| Lye (cleaning) | (oral-rat) 375 mg/kg | .036% |
| Propylene Glycol (cosmetics) | (oral-rat) 20,000 mg/kg | 2.0% |
| Whiskey (drinking) | (oral-rat) 14,200 mg/kg | 1.4% |
| Arsenic (poisons) | (oral-rat) 60.5 mg/kg | .006% |
We see that LD50 levels vary by factors of millions. LD50 data is one way we determine "degree" of toxicity.
LD50 data is more understandable when we look at what the animal data means on human exposure. One guideline to toxicity level is:
| Lethal Dose | Toxic Level |
|---|---|
| Less than 1.0 mg/kg | Dangerously toxic |
| 1 - 50 mg/kg | Seriously toxic |
| 50 - 500 mg/kg | Highly toxic |
| 500 - 5,000 mg/kg | Moderately toxic |
| 5,000 - 15,000 mg/kg | Slightly toxic |
| Over 15,000 mg/kg | Extremely low level toxicity |
A 150-pound person would have to ingest several quarts of a chemical to reach the 15,000 mg/kg level. We can see why materials that require these or greater quantities to show adverse effects are considered "extremely low toxicity."
Because their major component is water, Polywater® Cable Pulling Lubricants have extremely low toxicity. The ingestion of 50% of body weight (10-20 gallons) to reach a level with systemic effects is simply not realistic or possible.
Dermal Toxicity
Dermal toxicity can be quantified in the same way as ingestion (via LD50's), except the amount of material that must be absorbed through the skin to produce toxic effects is determined. Fortunately, the absorption of many substances through thick, exposed skin (hands, etc.) is rather slow. There are notable exceptions, including certain pesticides and steroids.
A different aspect of skin exposure is sensitivity or allergic reaction. Such reactions, which are specific to an individual, involve exposure to quantities much smaller than those required for systemic toxic effects. Almost any material can produce an allergy in a hypersensitive person. Skin patch tests are used to indicate the general skin irritation potential of various materials.
What You Can't See
Exposure to airborne dusts, mists, fumes, and vapors is difficult to determine and control in electrical construction. With their primary "evaporating" component being water, inhalation exposure is not a consideration in Polywater® Pulling Lubricant use. However, the solvent cleaners used in electrical splicing and terminating do, by their very nature, evaporate quickly and thus present a respiratory exposure.
The inhalation version of the oral lethal dose is called LC50--lethal concentration fifty--or the concentration required to produce a 50% test animal death rate. LC50 data involves not only the concentration of the substance in the air (usually in mg/m³ or ppm), but also exposure time, animal type, etc.
Because airborne inhalation is such a common industrial exposure, hygiene groups and/or government agencies (OSHA, NIOSH, ACGIH) have developed a set of airborne exposure limits called TLV's or PEL's (Threshold Limit Values or Permissible Exposure Limits). These exposure limits are given in ppm [parts per million (of air)] or pphm [parts per hundred million (of air)]. The most frequently used TLV limits are Time Weighted Averages (TWA's). They represent the maximum recommended airborne concentration under which most people can work (for an eight-hour day) without adverse health effects.
Cleaning Solvents With No TLV
TLV's for solvent cleaner/degreasers can be found on their MSDS's. But what if a cleaning solvent has "no TLV." Does this mean that unlimited exposure to its vapors is safe? Obviously not!! "No TLV" means that there is not enough information available for the industrial hygiene experts to "establish" a TLV, or a safe working level. Without such guidance, we must look for other data to determine reasonable exposure levels and potential respirator needs. Data from lethal concentration animal tests or TLV's from chemically similar materials might be considered.
Is Bigger Better?
Many people think that a material with a TLV of 500 ppm is "safer" than one with a TLV of 100 ppm. This is not necessarily true. For instance, if the TLV 500 ppm solvent evaporates quickly, vapor levels well above 500 ppm could easily occur in a vault or confined area. If the TLV 100 ppm solvent is slow to evaporate, the vault airborne concentration may never reach 100 ppm. Safe cleaning solvent use depends not only on the TLV, but also how much of the material evaporates, in what area, and with what kind of ventilation.
The Nose Knows?
Will smell tell us when we reach airborne concentration levels above a cleaner's TLV? Unfortunately no. Some cleaning solvents may have strong, irritating odors well below their TLV levels. However, others can have very little odor at concentration well above their permissible exposure limits. Unfortunately, it's a natural inclination to use cleaners that don't "smell" bad!!
Don't make the mistake of equating vapor level with odor. You cannot use a solvent cleaner safely based on whether or not people like its "smell."
One Way to Skin the TLV Cat
One popular package for American Polywater's HydraSol® Cable Gel Remover is a pouch containing a lint-free towel saturated with the unique, water-base HydraSol® Cleaner (catalog# HS-1). This "HS-1" offers convenience and time savings (plus the highly effective cleaner!).
Another advantage of the HS-1 package is vapor control! By determining the rate and amount of solvent evaporation from an HS-1, we have been able to develop a computer program to determine the vapor concentration in a confined work area. One such determination is shown below:
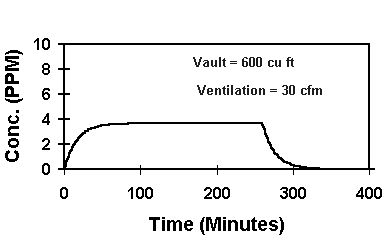
In this example, the vapor concentration from a single HydraSol® Towelette reaches about 4 ppm. This is less than 1% of the suggested 500 ppm TLV. The use of the HS-1 package allows you to engineer and control the exposure of your workers to solvent vapors. Bulk cleaner use (aerosol sprays, quarts, gallons, etc.) does not offer such exposure control.
Our Offer to You
If you would like details on a HydraSol® HS-1 exposure analysis (graph) for your specific situation, or a sample of an HS-1, please contact our Customer Service toll-free at 1-800-328-9384.
Summary
Not all that's important in chemical toxicology and safe use could be covered in this space. Your company's industrial hygienist is the expert in this area, and should be consulted with your specific problems.
You've seen the use of exposure and toxicity data from an MSDS to assess a workplace chemical's hazard. Such toxicity information can help you decide not only if you should use the chemical, but, more importantly, how you can safely use it.
View this article in PDF Format
For Subscriptions, Comments, or Questions contact: tteditor@polywater.com
Home |
About |
Site Map |
Literature |
Samples |
Links |
Reps |
Videos |
Pumps |
RFQ
Codes |
Engineering |
Shows |
Spotlight |
Newsletters |
MSDS |
Translations |
Contacts |
Jobs

11222 60th ST N | Stillwater, MN 55082-9310 USA
1-(651) 430-2270 (Voice) | 1-(651) 430-3634 (Fax)
1-(800) 328-9384 (Toll-Free US/Canada Only)
Copyright © 2001 - 2015 American Polywater Corporation | ![]() 6/5/15
6/5/15