| |
|||||||||
| |
|
|
|
|
|
|
|
||
| Home | About | Literature | Site Map | MSDS | Contact | Engineers | Shows | Links | Samples | RFQ | Catalog Numbers |

Part 1 and Part 2 of Electrical Cleaning Solvents discussed the EPA-mandated elimination of trichloroethane-based electrical cleaners. Properties of alternative solvents were examined.
Part 3 will look at another aspect of electrical cleaners: "vapor toxicity" and its control. We'll see how safe use of cleaners depends on evaporation rate and field use method.
Exposures
Field users' primary exposure to solvents is via inhalation (lungs). Because electrical cleaners evaporate, there are airborne vapors and a breathing exposure to the vapors.
Things we inhale can be toxicants or asphyxiants. Carbon monoxide (auto exhaust) and hydrogen cyanide (gas chamber) are examples of toxic gases. Gases like helium and nitrogen are asphyxiants. If they're not mixed with enough oxygen, they can smother us. Obviously, life-sustaining oxygen is neither a toxicant or asphyxiant. How do solvent vapors fit? How is their toxicity determined?
Toxicity of Vapors & Gases
What we need to know about airborne vapors is whether, and under what conditions, they're "safe." Industrial hygiene groups have answered this question, and established "safe working levels" for vapors or gases in air (40-hour-per-week exposure). These are called "Threshold Limit Values" (TLV's) or "Permissible Exposure Limits" (PEL's). TLV's describe the maximum recommended working level for an average human. They are "concentrations" in air, and are most often given as parts per million (ppm). One ppm is .0001 percent by weight in air.
TLV's of Airborne Gases and Solvent Vapors
The TLV's of the solvents in the following table vary from 2 to 1000 ppm (parts per million in air). Some solvents "do not have" a TLV (ND). This does not mean uncontrolled vapor exposure is safe. It means not enough data is available for experts to establish a "safe working level."
| GASES | TLV | SOLVENT VAPORS | TLV |
|---|---|---|---|
| Carbon Monoxide | 50 ppm | Ethanol | 1000 ppm |
| Methane | ND* | Methyl Ethyl Ketone | 200 ppm |
| Carbon Dioxide | 5000 ppm | Trichloroethane | 350 ppm |
| Ammonia Gas | 50 ppm | Perchloroethylene | 25 ppm |
| - | - | Carbon Tetrachloride | 2 ppm |
| - | - | Isohexane | 500 ppm |
| - | - | Octane | ND* |
| - | - | Citrus Solvent | ND* |
| * Not Determined | |||
What Creates Solvent Vapors?
The concentration of solvent in the air in a closed environment (vault, room, etc.) is determined by the following factors:
Individual examination of these factors will show how we can control them for safer solvent use.
American Polywater created a computerized model that estimates solvent concentration over time in an enclosure. The model bases projections on enclosure size, solvent evaporation rate, temperature, solvent type and amount, use method, and ventilation rate.
Amount Used
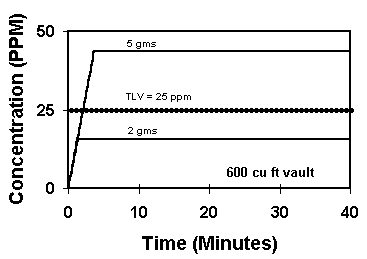
While trichloroethane had a relatively high TLV of 350 ppm, some of the chlorinated solvents that have been substituted do not. Graph #1 from the model shows two different amounts of perchloroethylene evaporated in a vault. Both amounts are fully evaporated in under 5 minutes. Perchlor's TLV of 25 ppm is shown as the dotted horizontal line. We see that over twice as much solvent results in over twice the air concentration. In this case, the higher amount has put perchlor above its maximum safe level of 25 ppm. 5 grams of solvent is less than .15 fluid ounces (not a lot).
TLV Effect
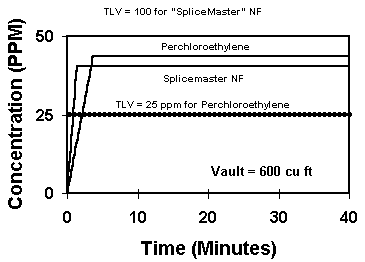
Graph #2 shows solvents with different TLV's. It compares perchlor with American Polywater's SpliceMaster® NF (5 grams of each). The two solvents evaporate at about the same speed, and the lines rise at roughly the same rate. Again, in 5 minutes, both solvents are fully evaporated. However, since SpliceMaster® NF™ has a higher recommended TLV (100) than perchlor, the resulting air concentrations are acceptable for the NF™, but not for the perchlor.
Evaporation Rate
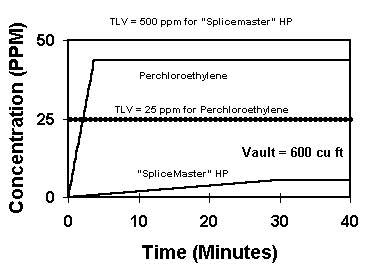
Graph #3 shows solvents with different evaporation rates. The perchlor evaporates more than 500 times faster than the SpliceMaster® Type HP™.
The slower drying SpliceMaster® HP™ releases very little vapor in the air over normal use time. The solvent rag is removed from the vault in 30 minutes. While the perchlor is fully evaporated and at roughly 45 ppm in 5 minutes, the SpliceMaster® HP™ is at less than 5 ppm after 30 minutes. As the graph shows, the removal of the used solvent rag from the vault at 30 minutes allows no release of vapor or increase in concentration.
Ventilation
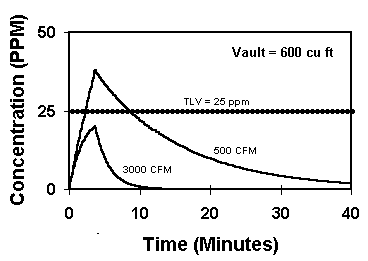
If solvent vapor levels are above safe working maximums, ventilation or protective breathing cannisters may be needed. Graph #4 shows two different levels of ventilation for 5 grams of perchlor evaporated. We see that the higher ventilation rate of 3000 CFM (with a distribution constant of 10) is required to keep the perchlor vapors below their TLV.
Engineering Safe Field Operations
The graphs indicate several ways to control solvent exposure.
The PEL-PAC® Solution
American Polywater's SpliceMaster® PEL-PAC® Package accomplishes the above objectives. Each package contains a precisely charged minimum amount of solvent on a lint-free, saturated towelette. The solvents have relatively high TLV's. The SpliceMaster® Solvents are available with different evaporation rates for specific field needs. PEL-PAC™ Kits come with or without pre-cut strips of non-conductive aluminum oxide sanding cloth for removal of stubborn semi-con shield residue. Additional dry towelettes are available by the box. American Polywater has the expertise to adapt the analysis shown to your specific situations. Just ask if interested.
Video and Back Issues
The "Electrical Cable Cleaning" video described in the last issue has been very well received. It covers field methods for splicing with slower drying cleaners, and much more. A flyer on the video and a complete list of back issues of "Technical Talk" are available by calling 1-800-328-9384
If you would like samples of the PEL-PAC® Packages, please call our Customer Service Department toll free at 1-800-328-9384.
View this article in PDF Format
Click here to view a Streaming Video on electrical cable cleaning
For Subscriptions, Comments, or Questions contact: tkeditor@polywater.com
Home |
About |
Site Map |
Literature |
Samples |
Links |
Reps |
Videos |
Pumps |
RFQ
Codes |
Engineering |
Shows |
Spotlight |
Newsletters |
MSDS |
Translations |
Contacts |
Jobs

11222 60th ST N | Stillwater, MN 55082-9310 USA
1-(651) 430-2270 (Voice) | 1-(651) 430-3634 (Fax)
1-(800) 328-9384 (Toll-Free US/Canada Only)
Copyright © 2001 - 2015 American Polywater Corporation | ![]() 6/5/15
6/5/15