| |
|||||||||
| |
|
|
|
|
|
|
|
||
| Home | About | Literature | Site Map | MSDS | Contact | Engineers | Shows | Links | Samples | RFQ | Catalog Numbers |
Background:
As telephone construction and maintenance operations eliminate ozone-depleting solvents, questions on the compatibility of replacements with various telephone components arise. One concern is the potential interaction with the "engineering plastics" often used in terminal blocks and connections. This report summarizes research into the compatibility of solvents with polycarbonate plastic.
Field surveys indicate that the telephone construction industry still uses considerable quantities of both CFC-113 (Freon) and Methyl Chloroform (1,1,1-Trichloroethane). The CFC-113 serves primarily as a contact cleaner, while the Methyl Chloroform is more broadly used as a general cleaning and degreasing solvent. Both solvents are Class 1 ozone depleters and are under an accelerated elimination schedule under the 1990 Clean Air Act and subsequent administrative actions.
Purpose:
This research studies the stress cracking of polycarbonate plastic by a variety of solvents and solvent blends. The results of this research can be used to develop targeted replacements for 1,1,1-Trichloroethane and CFC-113, replacements that have equivalent or better compatibility with polycarbonate plastics.
Procedure:
The polycarbonate used was a colorless, transparent Makrolon 2600 from Mobay Corporation. This polycarbonate is not highly solvent resistent, and should show greater stress cracking tendency to develop "conservative" cleaning solvents. The plastic was received as injection-molded plaques, 1/8-inch thick. These were cut into bars, 2½-inch by ½-inch. The polycarbonate bars were then annealed in an air circulating oven for 24 hours at 120°C, as preparation for testing.
Samples were bent in a three-point fixture modified from one described in reference [1]. (See figure 1). Percent strain, E, is related to the thickness of the bar, T, and the radius of curvature of the fixture, R, using the following formula. (See reference [2]).
E (%) = T / 2R+T x 100
The fixture consists of two posts 2 inches apart and a machine screw to bow (stress) the polycarbonate bar. A micrometer is used to measure deflection of the polycarbonate bar. Advancing the screw produces a radius of curvature (R) on the outer surface of the plastic. (See Figure #1).
Displacement, d, is related to the radius of curvature of the fixture, R, by:
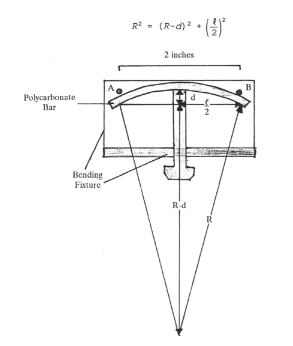
Figure #1
3-point bending fixture produces bow (stress) in the polycarbonate bar.
The following strains are calculated for various displacements, d.
| Mils Displacement, d | % Strain, E |
|---|---|
| 16 | 0.2 |
| 24 | 0.3 |
| 40 | 0.5 |
| 57 | 0.7 |
| 73 | 0.9 |
| 98 | 1.2 |
Several different methods are described for rating the chemical resistance of polycarbonate to solvents, or conversely, the action of solvents on polycarbonate. In reference [1], a pass/fail test is recommended. A solvent that caused stress cracking at a .5% strain and less in any of the samples fails the test. Reference [2] uses the following to describe the chemical resistance of polycarbonate to a solvent.
a) Resistant - 1.2% strain limit or greater without cracking.
b) Limited Resistance - 0.6% to 1.0% strain limit without cracking.
c) Non-Resistance - 0.4% strain limit or less without cracking.
"Strain limit" is the greatest percent strain where no stress cracking occurs. It would appear that a strain limit of at least 0.5 to 0.6% is desirable for a solvent that must contact polycarbonate. However, this study compares cleaners and no direct correlation to actual performance on field parts is made.
Application Method Results:
Another variable to be considered in polycarbonate stress crack testing is "solvent application method." In a set of experiments, certain cleaning solvents were applied to stressed (0.5%) polycarbonate bars via a wipe, a spray, and a soak immersion.
All these methods mimic possible degree of contact in the field. The test results are summarized in Figure #2 below. A 15-minute soak with air drying (no water rinse or mechanical drying performed)was chosen as the preferred testing method for the rest of the study. The data show this is the most severe test method and should conservatively predict results in the most difficult field exposure. The 15-minute soak is also the most efficient and reproducible method.
| Solvent | Application Method (E = 0.5%) | ||
|---|---|---|---|
| Spray | Wipe | Soak | |
| Trichloroethane | 2 | 4 | 0 |
| Polywater® Formulation I | 6 | 10 | 4 |
| Cyclic Hydrocarbon | 6 | 8 | 4 |
| Polywater® Formulation J | 10 | 10 | 8 |
Figure #2
Scale Rating:
0 - Polycarbonate dissolved
2 - Immediate crack of bar into two pieces
4 - Severe cracking - approximately 1/3 of surface
6 - Slight cracking or crazing on the surface
8 - Very slight cracking - 1-2 surface cracks
10 - No effect on polycarbonate
Multiple Solvent Strain Limit Results:
The strain limit results on a great variety of solvents are shown in Figure #3. The solubility parameters cited were obtained from solvent literature. In the cases where solvents are blended, solubility parameters were calculated using a weighted arithmetic mean (gram weight) for each solvent.
The results show several significant things:
| Solvent | Hildebrand Solubility Parameter | Percent Strain Limit |
|---|---|---|
| Polywater® Formulation A | 7.0 - 7.3 | >0.9 |
| Polywater® Formulation B | 7.2* | >0.9 |
| Polywater® Formulation C | 7.4 | >0.9 |
| Polywater® Formulation D | 7.5 | >0.9 |
| Polywater® Formulation E | 7.6* | 0.7 |
| Polywater® Formulation F | 8.0* | 0.5 |
| Cyclic Hydrocarbon | 8.1 - 8.6 | 0.3 |
| Cyclic Hydrocarbon | 8.2 | 0.7 |
| Commercial Formulation | unknown | 0.7 |
| Polywater® Formulation G | unknown | 0.5 |
| Halogenated Solvent A | 7.0* | 0.7 |
| Halogenated Solvent B | 8.2* | 0 |
| Halogenated Solvent C | 7.4* | <0.3 |
| Halogenated Solvent D | 8.2* | 0 |
| Halogenated Solvent E 1,1,1-Trichloroethane | 8.5 | 0 |
| Polywater® Formulation H | unknown | 0.7 |
| Oxygenated Solvent A | 8.4 | 0 |
| Oxygenated Solvent B | 8.9 | 0 |
| Oxygenated Solvent C | 9.1 | 0 |
| Oxygenated Solvent D | 9.9 | 0 |
| Oxygenated Solvent E | 10.0 | 0 |
| Oxygenated Solvent F | 11.4 | 0.7 |
| Oxygenated Solvent G | 12.0* | 0.5 |
| Oxygenated Solvent H | 14.5 | 0.7 |
| Oxygenated Solvent I | 10.9* | 0 |
| Polywater® Formulation I | 11.0* | 0.3 |
| Commercial Formulation | unknown | <0.3 |
| Polywater® Formulation J Solvent-Water Emulsion | unknown | 0.3 |
| Polywater® Formulation K Water-Base | unknown | 0.5 |
| Commercial Formulation Water-Base | unknown | 0.9 |
Figure #3
* Estimated
Discussion - Solubility Parameter Relationships:
Graph #1 shows a plot of the solubility parameters of various solvent and solvent blends versus minimum strain percent at which stress cracking of the polycarbonate occurs. Solubility parameters quantify and predict the molecular solvent properties of organic materials. Solubility parameters have been used in previous research to predict encapsulant compatibility with polycarbonate [3]. The extension of this theory to various solvents and solvent blends seems valid. The data shows that solubility parameters are good predictors of maximum percent strain limit for polycarbonate stress cracking.
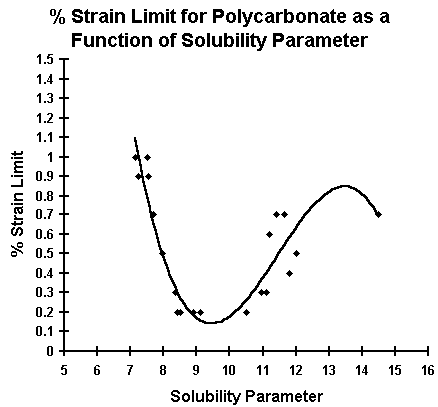
Graph #1
Polycarbonate has a reported solubility parameter of 9.3 to 10.3. The Makrolon 2600 appears particularly sensitive (percent strain limit less than 0.5) to solvents with solubility parameters ranging from 8.0 to 11.2.
Due to stronger intermolecular forces, solubility parameters are most meaningful for non-polar solvents [4]. We believe this is reflected in the high variability in polycarbonate stress cracking results for alcohols and other polar solvents (solubility parameter greater than 11.2).
1,1,1-Trichloroethane, with a solubility parameter of 8.5, has been found to actually dissolve polycarbonate. It could be possible that trichlor has been used without noticeable effect only because the evaporation rate is so quick and contact is incidental and of very short duration. It can be expected that any extended contact with Trichloroethane, such as a solvent-soaked rag, would have an effect on polycarbonate.
A cleaner can be chosen or formulated solely to reduce the likelihood of stress cracking polycarbonate. However, this approach does not take a solvent's "cleaning effectiveness" into consideration. The efficacy of a cleaner is directly related to its solubility parameter and the solubility parameter of what's being cleaned or dissolved.
We believe that a grease or contaminant in the same solubility range as polycarbonate, 8.0 to 11.2, will cause the greatest concern. To remove this contaminant it may be necessary to use a solvent that could cause stress cracking. However, if not removed, the grease itself could cause stress cracking.
When the end use requires a cleaner with a solubility parameter in the 8.0 to 11.2 range, proper cleaning techniques would require a minimum amount of solvent cleaner and a wipe with a clean drying towelette to minimize contact time.
Solvents should be chosen based on both their ability to clean efficiently and their compatibility with surrounding parts and materials. When these two are in conflict, more detailed testing on final parts, the specific contaminant, and various cleaning methods is recommended.
More detailed product literature is available on American Polywater's SpliceMaster® cleaning products line. Call or write with specific questions or problems.
Prepared by: Sheri Dahlke - Product Development Chemist
References:
[1] Bell Communications Research, Wasp and Hornet Spray Technical Reference, TR-620-23352-84-03, Issue 1, August 1984, Section 5.4 Parts B, C, and D.
[2] Mobay Corporation, Plastics and Rubber Division, Chemical Compatibility Test for Unreinforced Thermoplastic Resins, 1989.
[3] Croft et al., TelComm Products Division Laboratory/3M, Austin, TX; "A Novel Non-Polyurethane Re-entereable Encapsulant Compatible with Both Filled and Polycarbonate Connectors" from International Wire & Cable Symposium Proceedings, 1987.
[4] Robert C. Weast, ed., CRC Handbook of Chemistry and Physics, CRC Press, Inc., Boca Raton, Florida, 1982, p. C-732 - 734.
Click here to Add Your Name To Our Mail List
Home |
About |
Site Map |
Literature |
Samples |
Links |
Reps |
Videos |
Pumps |
RFQ
Codes |
Engineering |
Shows |
Spotlight |
Newsletters |
MSDS |
Translations |
Contacts |
Jobs

11222 60th ST N | Stillwater, MN 55082-9310 USA
1-(651) 430-2270 (Voice) | 1-(651) 430-3634 (Fax)
1-(800) 328-9384 (Toll-Free US/Canada Only)
Copyright © 2001 - 2015 American Polywater Corporation | ![]() 6/5/15
6/5/15